Efficacy Of Covishield And Vaccine Race

Dr. Rabindra Pandey
The development of the much-awaited anti-COVID-19 vaccine within a year of the pandemic outbreak is undoubtedly a scientific miracle. In the current scenario, the production of vaccines is limited but the demand is unlimited. Thus, it is a big success for Nepal to launch the vaccination drive. India had provided one million doses of Covishield vaccine in grant to Nepal. It is invaluable medical support to Nepal as the vaccine has protected the frontline workers of various professions.
The Covishield vaccine was developed by a research team from Oxford University and the British-Swedish pharmaceutical company AstraZeneca. Produced by Serum Institute of India, this vaccine uses a harmless adenovirus found in chimpanzees. It transports the surface protein of SARS-CoV-2 to human cells, triggering an immune response against the coronavirus. A single standard dose of Covishield provides 76 per cent protection against symptomatic COVID-19 in the first 90 days after vaccination. This vaccine is effective against a new variant of virus detected in the UK.
Virus Control
Various studies have found that Covishield can be 82.4 per cent effective in those who take the second jab in the interval of 12 weeks. If the two doses are administered in an interval of fewer than six weeks, the efficacy of the vaccine will only be 54.9 per cent. Moreover, the researchers have found out that after a single dose of vaccine, overall chances of contracting the virus has dropped by 67 per cent, raising hopes that the vaccine will drastically control the virus transmission, thereby reducing the number of infected people.
The World Health Organisation (WHO) has recommended the use of the Covishield vaccine for everyone aged 18 and over, even if there are coronavirus variants present. The WHO stated that people, who have already contracted COVID-19, can be vaccinated but they can delay their own shots for up to six months from the day of infection so that those in the urgent need of protection will be inoculated. However, people with a history of a severe allergic reaction to any ingredient of the vaccine should not take it.
Covishiled generates the immune response inside the body and those responses are protective against the virus. Oxford and AstraZeneca have agreed to provide the vaccine to buyers for the US $2-3 per dose. Covishield vaccine is cheaper and easier to make in bulk than the RNA vaccines from Pfizer and Moderna. It also does not need to be stored at temperatures as low as the RNA vaccines, one of which must be kept at -70 degrees Celsius until shortly before it is administered. Similarly, the WHO's decision paved the way for the UN-backed COVAX scheme to start shipping the doses of the Oxford/AstraZeneca vaccine around the world to lower-income countries. The vaccine, which is set at not-for-profit prices and only needs ordinary refrigeration at 2 to 8 degrees Celsius, has raised the hope for many countries to vaccinate their health workers and vulnerable citizens.
But the Oxford/AstraZeneca vaccine also drew controversies after some European countries, including France and Germany said they would not give the vaccine to older people. In recent days, leaked results from a small, unpublished study involving younger people show minimal protection (only about 10 per cent) against mild to moderate illness caused by the South African variant of the virus. As a result, South Africa has stopped the vaccination drive.
Similarly, Germany's vaccine commission, STIKO, has advised that AstraZeneca only be given to people aged 64 and under. The commission cited a lack of data regarding the vaccine's effectiveness for older people. Meanwhile, according to AstraZeneca, the vaccine's simple supply chain and no-profit pledge would make it more affordable, but the exact price of a dose of the AstraZeneca vaccine is not clear. AstraZeneca and BioNTech-Pfizer both have made agreements with COVAX, a global initiative for providing low-cost vaccines to low- and middle-income countries
The COVID-19 vaccines that have been approved for use make the body produce antibodies against the spike protein of the original strain of the coronavirus. But now, the antibodies fight viruses whose spike proteins they do not fully recognise. The shape of the virus has slightly changed, but the response that one is making is based on the original one. This means that no antibodies attach to the mutated part of the spike protein, and the virus can still attach itself to a human cell. But the antibodies will block the parts of the virus that it does recognise, so it still provides some protection.
A study of the Moderna vaccine found that it was slightly less effective against the UK variant, but the neutralising levels were still above those expected to be protective. A study of the BioNTech-Pfizer vaccine also found that the jab was slightly less effective against the B.1.351 variant. The head of the Oxford research group, Sarah Gilbert, told the BBC that the vaccine should still protect against severe disease. But at the same time, she said developers are working on a modified vaccine to combat the South African variant. This would likely be ready in autumn, she said. “Even if the efficacy drops down to as low as 10 per cent, it is still the right thing to do to immunise older adults because of the higher risk of severe disease and mortality in that age group,” she said.
On January 25, German economic newspaper Handelsblatt claimed: “The AstraZeneca vaccine apparently has an effectiveness of only 8 per cent in the elderly. The government’s vaccination strategy is shaky.” The news piece caught the attention of people globally only to be rebuffed later. An AstraZeneca spokesperson dismissed the report as “completely incorrect.”
Investment
Many countries have been making huge investment in research and development of the vaccine. It has sparked a kind of competition among the vaccine companies of different countries. Competition can lead one company to confuse another company's vaccines. Therefore, we must believe in nationally and internationally recognised vaccines without following rumours and illusions. However, the vaccine is not enough to end the pandemic. People must continue to follow public health regulations like maintaining social distancing, wearing masks, and washing their hands regularly.
(Dr. Pandey is a public health expert and have been involved in study and campaign against COVID-19)
Recent News

Do not make expressions casting dout on election: EC
14 Apr, 2022
CM Bhatta says may New Year 2079 BS inspire positive thinking
14 Apr, 2022
Three new cases, 44 recoveries in 24 hours
14 Apr, 2022
689 climbers of 84 teams so far acquire permits for climbing various peaks this spring season
14 Apr, 2022
How the rising cost of living crisis is impacting Nepal
14 Apr, 2022
US military confirms an interstellar meteor collided with Earth
14 Apr, 2022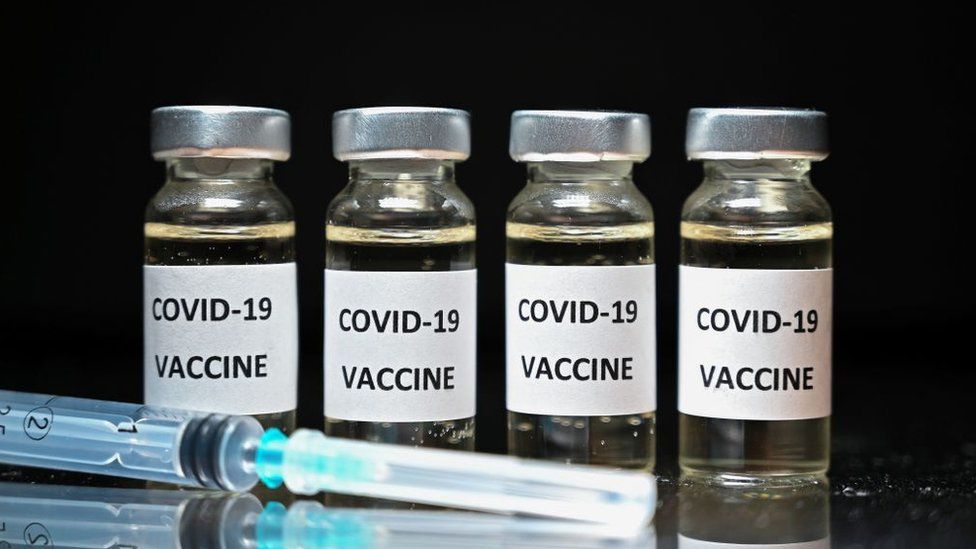
Valneva Covid vaccine approved for use in UK
14 Apr, 2022
Chair Prachanda highlights need of unity among Maoist, Communist forces
14 Apr, 2022
Ranbir Kapoor and Alia Bhatt: Bollywood toasts star couple on wedding
14 Apr, 2022
President Bhandari confers decorations (Photo Feature)
14 Apr, 2022