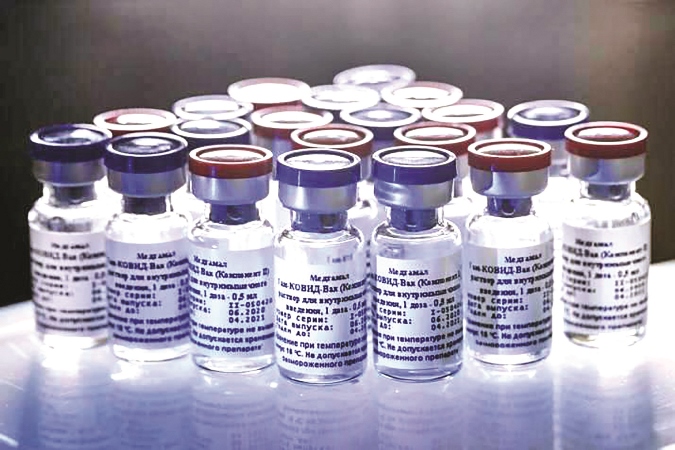
Race For Coronavirus Vaccines
Four years. That’s the fastest a vaccine has ever been developed -- and most take 10 to 15. But scientists are now racing to do it in under one.
Dozens of research teams around the world are working to develop a vaccine for SARS-CoV-2, the virus that causes Covid-19, using a mix of established techniques and new technologies.
Funding for a vaccine has never been greater, with billions of dollars pouring in from around the world to make a product that could help to control the pandemic -- but the US, China and Europe have invested the most.
Before even the most vulnerable groups can get a shot in the arm from their family doctor, however, a lot of work needs to be done -- and a lot of deals need to be made.
Vaccines In 2021?
Scientists are hoping to deliver a vaccine that protects against Covid-19, and its transmission, by early 2021. In order to do that, the development process has been rapidly accelerated.
A vaccine must go through multiple stages before being green lit for use. An initial research and development stage is followed by a series of pre-clinical and clinical trials (consisting of three phases), and typically each step can take two years or more to complete.
But in the race to stop the coronavirus, some of those steps are being combined, or skipped altogether, to speed up the process.
Most of the candidates in human trials have eitherAmerican, European or Chinese financial backing, and some experts believe it will be regulators in one of those places that will be the first to approve a safe and effective vaccine. American biotechnology firm Moderna was the first in the world to kick off human trials on March 16, just two months after the genetic sequences for SARS-CoV-2 were identified.
Now there are six vaccines in the last stage of human trials (Phase 3) before they seek approval: Three from China, two developed by state-owned Sinopharm and one from private Chinese firm Sinovac Biotech; one from the United Kingdom, produced in partnership between the University of Oxford and AstraZeneca; and two from the US, one by pharmaceutical giant Pfizer, in addition to the candidate by Moderna.
To reach this late stage of trials just six months after discovery is remarkably fast -- it would normally take at least six years, according to Professor Adrian Hill, director of the Jenner Institute at Oxford University.
In a bid to get something out as soon as possible, some countries are pushing forward vaccines before their efficacy has been proven in Phase 3 trials. China has already done it, approving an experimental vaccine for use by its military in late June.
Russian Vaccine
Now Russia is touting a vaccine, despite concerns that corners have been cut in its development. On August 11, President Vladimir Putin announced the registration of a vaccine named Sputnik V, the first in the world to be approved for public use. Russia hasn’t released scientific data on its testing, however, and the vaccine was approved the day before the start of Phase 3 trials -- the last and most critical step in the process.
“I hope that the Russians have actually, definitively proven that the vaccine is safe and effective. I seriously doubt that they've done that,” Dr. Anthony Fauci, the director of the US National Institute of Allergy and Infectious Diseases, told ABC News’ Deborah Roberts.
When Americans hear announcements from countries like Russia or China about vaccine development, Fauci said they should remember that the US has very different standards in place.
AstraZeneca has said it reached agreements with several governments -- including the US and UK -- and other organizations to produce at least 2 billion doses of vaccine, with the first deliveries starting as early as September. The Biomedical Advanced Research and Development Authority (BARDA), a branch of the US Department of Health and Human Services, is injecting billions of dollars into companies to ramp up vaccine development and manufacturing, including AstraZeneca. It has also inked deals for doses with Novavax, Pfizer-BioNTech, Johnson & Johnson, Moderna and Sanofi and GSK’s joint effort.
This speed is one of the reasons many citizens are wary of getting vaccinated. A recent CNN poll found only 66% of Americans would get a Covid-19 vaccine should one become available. And, even if everyone does take the vaccine, it almost certainly won’t be 100% effective.
Effectiveness of Vaccines
Many vaccines are not even close to 100% effective. For example, the world’s first malaria vaccine — RTS,S or Mosquirix -- was rolled out across Africa last year, despite offering only 39% protection against malaria in children aged 5-17 months.
This was considered to be worth it in countries with high rates of the disease when used alongside other interventions.
In comparison, the US Food and Drug Administration have said that a coronavirus vaccine would have to protect at least 50% of vaccinated people to be considered effective. It’s thought that level of efficacy can help stop transmission, especially in combination with other treatments and prevention measures.
Some experts believe that the virus will never really go away and that it may instead become a typical, more manageable infection like influenza.
Others warn that the coronavirus vaccines that cross the finish line first may not necessarily be the best, and that in the end we are likely to be using multiple vaccines to control the pandemic. This was the case for the Ebola epidemic, where a number of investigational vaccines were made available for use after proving to be effective in field trials.
Quest To Vaccinate World
The quest to vaccinate the world is also exposing gaps in the pharmaceutical supply chain. For example, the world has neither enough glass vials for the vaccine, nor the capacity in factories to fill and package them, experts say.
But countries have released extensive funds to fill the void.
The US has already allocated more than $1.5 billion for domestic manufacturing and distribution in order to ensure a vaccine can be produced to scale.
The rush to secure supplies is inflaming geopolitical issues too.
The World Health Organization (WHO) has encouraged countries to cooperate in order to ensure fair global access, but some experts believe national interests will get in the way.
Robinson says a precedent was set during the 2009 H1N1 influenza pandemic, also known as swine flu, which the CDC estimates killed as many as 575,000 people worldwide (31 times more than numbers reported by WHO). When a vaccine became available, wealthier countries bought up the supplies, leaving poorer nations at the back of the line, according to WHO and public health experts.
Fortunately, that strain of H1N1 was much milder than experts had predicted and some older populations showed some immunity to it, meaning the vaccine didn’t play a key role in ending the pandemic.
But Covid-19 is in a different league and is showing no signs of a natural decline.
In the hopes of avoiding a repeat of what happened in 2009, WHO launched the Access to Covid-19 Tools’ (ACT) Accelerator on April 24 in which countries agree to ensure “all people have access to all the tools to prevent, detect, treat, and defeat Covid-19.” But the US and China didn't sign it.
Just over a month later, President
Donald Trump announced America’s withdrawal from WHO.
And in another worrying signal, in late June, the US federal government bought up nearly all the global supply of remdesivir available through September -- the first drug shown to be effective in treating severe cases of Covid-19.
It’s feared that the remdesivir agreement, which prioritizes American patients, will serve as a blueprint for distribution of future treatments and vaccines.
Access To Poor
But the question remains: Will leaders -- the world over -- be able to respond and buy the vaccine for their own populations if one of the big three efforts succeeds? And will poorer nations once again be left behind?
It is this concern that prompted Dr. Seth Berkley, CEO of Gavi, the Vaccine Alliance, to call for an international accord to ensure poorer countries get access to the vaccine.
The best-case chance of getting a vaccine, according to Berkley, is adopting the collaborative approach that was used in the race for an Ebola vaccine -- a process which began in the Canadian Public Health laboratory, was transferred to an American biotech firm, then an American multinational company, and finally to a German company for manufacturing. Those efforts, combined with those from organizations such as Gavi and the International AIDS Vaccine Initiative, aim to deliver safe and effective vaccines to everyone who wants one.
Countries Racing For Vaccines
United States
The US government has taken an “America First” approach to finding a vaccine. Its Operation Warp Speed initiative is a sprawling effort that’s been likened to the US mission to get a man on the moon. It was launched on May 15 with the aim of delivering 300 million “safe, effective” doses by January 2021.
The mission has selected eight of the most promising vaccine candidates (with one still yet to be named) and supercharged them, supported by the full might of the US government machine -- from manufacturing in federal facilities to distribution by the US Department of Defense.
More than $12.3 billion has already been dedicated to vaccine efforts -- $10.8 billion for vaccine development and procurement, and $1.5 billion for manufacturing and distribution.
Europe
The European Commission began extensive funding programs for vaccine research and development at the beginning of the outbreak in January -- and has pledged €350 million ($412 million) so far.
Under its research and innovation program, known as “Horizon 2020,” and with financial help from the European Investment Bank, loans were also set up to aid vaccine efforts.
As part of that funding, the EU has lent €75 million ($88 million) to German biotech company Curevac and €100 million ($117 million) to BioNTech, another German firm -- both of which have vaccine candidates in human trials.
The EU has also begun exploring options for procurement of a future vaccine, pledging to use a significant part of a €2.7 billion ($3.2 billion) emergency fund to bulk buy doses for all EU members and help subsidize costs for developers in exchange for doses.
So far, the EU has backed, or provisionally agreed to back, the purchase of at least six vaccines.
China
If the US and Europe have merely hinted that they’ll prioritize their people first for vaccines, China has made no secret of its plan to do exactly that.
President Xi Jinping told the World Health Assembly, the decision-making body of WHO, in May that Beijing would help provide a vaccine globally, but only after it had been deployed in China.
China has a shorter history of vaccine expertise compared to the US and Europe, but it has size on its side. It is the world’s largest producer and consumer of vaccines and can supply more than 1 billion doses of a vaccine annually from 40 manufacturers across the country, according to the China Human Vaccine Industry Report, 2018-2022.
Indian Vaccine
India’s first indigenously developed COVID-19 vaccine, COVAXIN, could be available by the end of this year. Recently, it was reported that the Oxford’s COVID-19, dubbed Covishield in India, is likely to be the first shots available for Indians by the end of 2020 if trials succeed.
Apart from the COVAXIN jointly developed by Bharat Biotech and ICMR, the Zydus Cadila’ ZyCoV-D vaccine and Oxford-AstraZeneca’s candidate being produced by the Serum Institute of India are being tested across the country. According to the report, the government is considering an initial order of about 50 lakh doses of coronavirus vaccine for certain groups of people such as frontline workers and army personnel.
Multiple Stages
Pre Clinical:
Before testing on humans, researchers usually give the vaccine to animals to assess safety and see if it triggers an immune response. But for some coronavirus vaccines, researchers have been able to speed up normal protocol by testing animals and humans in parallel.
Phase 1:
In the first stage of clinical trials, the vaccine is given to a small group of people (usually between 10-50) to check it’s safe.
Phase 2:
Then it's tested on several hundred people to further gauge safety, any potential side effects, the immune response and dosage. But a number of coronavirus vaccines are in simultaneous Phase 1/2 trials, which means they’re being tested for the first time on hundreds of people.
Phase 3:
In the last stage, scientists give the vaccine to thousands of people across different ages and locations and see how many contract the virus, compared to those who received a placebo. This determines how good it is at reducing new infections -- its efficacy.
Implementation:
Regulators then review the trial results to decide whether to approve the vaccine for use, licensing and large-scale manufacturing. Many vaccines also undergo Phase 4 studies after they are approved and licensed.
There are currently 29 vaccines being tested in multiple human trials, running simultaneously around the world. Among these, six vaccines are being tested in seven Phase 3 trials. Progress on a vaccine for Covid-19 has been rapid compared to other viruses, with human trials starting just 67 days after the outbreak began.
Courtesy: CNN/ Agencies
Recent News

Do not make expressions casting dout on election: EC
14 Apr, 2022
CM Bhatta says may New Year 2079 BS inspire positive thinking
14 Apr, 2022
Three new cases, 44 recoveries in 24 hours
14 Apr, 2022
689 climbers of 84 teams so far acquire permits for climbing various peaks this spring season
14 Apr, 2022
How the rising cost of living crisis is impacting Nepal
14 Apr, 2022
US military confirms an interstellar meteor collided with Earth
14 Apr, 2022
Valneva Covid vaccine approved for use in UK
14 Apr, 2022
Chair Prachanda highlights need of unity among Maoist, Communist forces
14 Apr, 2022
Ranbir Kapoor and Alia Bhatt: Bollywood toasts star couple on wedding
14 Apr, 2022
President Bhandari confers decorations (Photo Feature)
14 Apr, 2022