Plasma Therapy Requires Utmost Care
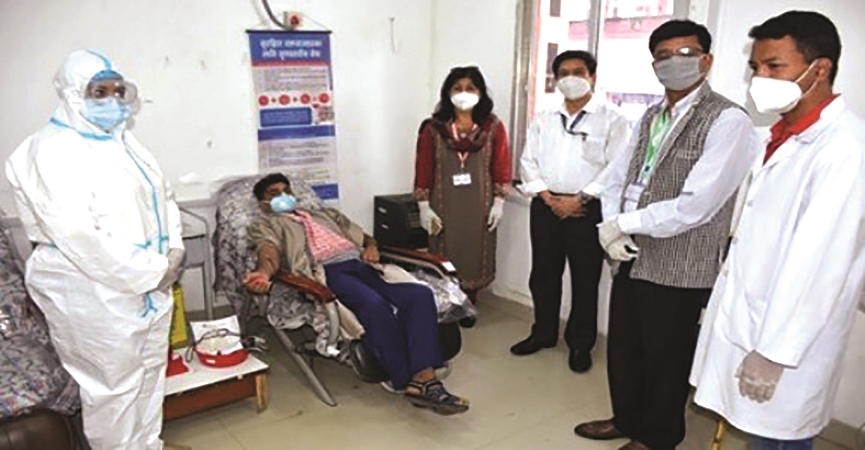
Dr. Prakash Budhathoky
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which causes COVID-19, is proving to be a mysterious virus even though knowledge about the disease is evolving every day. The guidelines for the management of patients are developed and regularly updated based on the latest research. This has created several challenges, one being the discrepancy between trial findings and their clinical application.
There are no specific drugs for the treatment of COVID-19 till now and drugs are being repurposed as a potential treatment for the disease. However, the multi-country Solidarity Therapeutic Trial, spearheaded by the World Health Organisation (WHO), found that remdesivir did not reduce the mortality rate among the hospitalised patients with COVID-19.
Convalescent Plasma
The other drugs tested alongside were hydroxychloroquine, lopinavir/ritonavir and interferon, but they also did not help reduce mortality among the patients. The results were surprising as trials by the National Institute of Allergy and Infectious Diseases, published on October 8, 2020, in the New England Journal of Medicine had shown that recovery was faster with remdesivir in hospitalised patients with COVID-19.
On October 22, remdesivir became the first treatment for COVID-19 to receive the USA based Food and Drug Administration (FDA) approval for use in adults and children aged 12 years or older.
Another line of treatment is convalescent plasma, which is experimental and has received regulatory approval for its use in COVID-19 patients who do not respond to standard treatment. FDA accorded its emergency use authorisation as a potentially promising treatment.
Convalescent plasma is plasma collected from persons who have recovered from COVID-19 by the process of apheresis. This is a medical procedure where whole blood is removed from a donor or patient and separated into individual components so that only one component can be removed. Plasma therapy has been used as emergency intervention in many outbreaks, including the Spanish Flu, SARS, MERS and Ebola. Except for Ebola, it has been beneficial when administered early in the course of the disease.
Convalescent plasma contains protective, neutralising antibodies to the coronavirus. When transfused into a patient, these antibodies have a direct antiviral action by suppressing virus replication and may hasten recovery. The presence of anti-inflammatory cytokines, clotting factors, natural antibodies and other undefined proteins in the plasma may further enhance anti-inflammatory and immune-modulatory response.
Convalescent plasma can be collected anytime between 14 and 28 days following COVID-19. Anyone who has recovered from COVID-19 and completed 28 days after the completion of treatment or home isolation and is between 18 to 60 years is eligible to donate their blood plasma. The potential donor must have documented COVID-19 infection and tested negative for the virus.
The women who have been pregnant cannot donate plasma. This is because pregnant women develop anti-human leukocyte antigens (HLA) antibodies against paternal antigens. These anti-HLA antibodies usually do not affect the woman or her subsequent pregnancies. But when plasma containing HLA antibodies is transfused to a person with lung damage, as generally occurs in COVID-19, this may lead to transfusion-related acute lung injury (TRALI). COVID-19 induces a hyper-coagulable state in the body. Plasma contains pro-coagulants and hence must be used with caution in patients.
Clinical Management
The clinical management protocol for COVID-19 includes convalescent plasma as an investigational therapy "for consideration in patients with the moderate disease who are not improving (oxygen requirement is progressively increasing) despite the use of steroids".
The dose ranges from four to 13 ml/kg. These include- ABO compatibility and cross-matching of the donor plasma and neutralising titer of donor plasma should be above the specific threshold while recipient should be closely monitored for several hours post-transfusion for any transfusion related adverse events and the use should be avoided in patients with IgA deficiency or immunoglobulin allergy.
The effectiveness of convalescent plasma came into question following the publishing of the PLACID trial by the ICMR. The trial was conducted in 39 hospitals, both public and private, across India on 464 adults 18 years and above with confirmed, moderate COVID-19. The results showed that while it was safe, there was no survival benefit of convalescent plasma over the best standard of care. However, the trial did show that plasma therapy improved symptoms like difficulty in breathing and fatigue with faster virus clearance.
Clinical practice today is undergoing a shift from evidence-based medicine to person-centric or patient-centric care. Person centric medicine recognises that every person has a distinct set of characteristics that distinguishes them from the other. Every patient has to be managed differently for the best possible outcome.
Individual response to drugs may vary, the side effects may vary. The treatment has to be tailored to each patient taking into consideration their characteristics, culture, personal preferences, expectations, etc. All these variables need to be taken into account when formulating a treatment plan for the best response and maximum safety of that patient.
Research And Guidelines
New research and guidelines follow suggestions and recommendations, which are evidence-based. While they do standardise treatment for any given condition, they are not without limitations. They cannot be generalised and extrapolated to all patients alike as each patient is unique and hence, treatment has to be individualised.
The first and till now COVID-19 patient in the country to receive convalescent plasma therapy was given this treatment on compassionate grounds upon request from his family when there was no improvement with standard treatment. If trials and /or guidelines “dictate” or “decide” treatment, this impinges on the professional autonomy of a doctor. The decision to use a particular treatment modality, and when to use it, is best left to the treating clinician.
(Budathoky is Central Treasurer at Nepal Medical Association)
Recent News

Do not make expressions casting dout on election: EC
14 Apr, 2022
CM Bhatta says may New Year 2079 BS inspire positive thinking
14 Apr, 2022
Three new cases, 44 recoveries in 24 hours
14 Apr, 2022
689 climbers of 84 teams so far acquire permits for climbing various peaks this spring season
14 Apr, 2022
How the rising cost of living crisis is impacting Nepal
14 Apr, 2022
US military confirms an interstellar meteor collided with Earth
14 Apr, 2022
Valneva Covid vaccine approved for use in UK
14 Apr, 2022
Chair Prachanda highlights need of unity among Maoist, Communist forces
14 Apr, 2022
Ranbir Kapoor and Alia Bhatt: Bollywood toasts star couple on wedding
14 Apr, 2022
President Bhandari confers decorations (Photo Feature)
14 Apr, 2022